In this article, we will talk about the Identifying food fraud, food quality, and ash of dried fruits. One of the general indexes of product quality is measuring total ash. Ash is the remained mineral after burning total organic materials. Therefore, the high content of ash shows the low quality of nutrition for food frauds, because the unnatural ash content after burning shows the presence of non-organic and unnatural materials such as sand, gravel, soil, starch, and so on. Thus, the total ash test is used to detect the quality and nutrition value of food products.
Furthermore, some minerals are not digested in the human or animal body, which can be dangerous to health. Because foods must be soluble in stomach acid. These materials that can be there by the food frauds and adding foreign materials is identified as a quality evaluation index using “acid insoluble ash test”. It means the more acid insoluble ash leads to less digestion of that food and its impurities increase. Total ash and acid insoluble ash tests are some of the most important tests in the food industries which are described later.
Total ash is the remained mass of dried fruits after being incinerated in 550±25 °C in the specific condition based on mass percentage and according to Iran national standard No. 1197.
Acid insoluble ash is obtained from the total ash and is the non-reacted part of the reacted ashes with hydrochloric acid and is stated as mass percentage. The methodology is to first calculate the total ash.
Read more:
Measurement of dried fruit and nut moisture by analyzer scale

How to calculate total ash in dried fruits to prevent food fraud
1- Laboratorial supplies:
- Distilled grade 3 water according to the national Iran national standard No. 1728
- Flat-bottom crucible with a capacity of 50 to 100 ml made by platinum, quartz, porcelain, or other immutable materials based on test conditions.
- Heater
- Electric muffle furnace with a temperature capability of 550±25 °C
- Desiccator
- Scales with an accuracy of 0.0001 grams
- Ben Murray
2- Sampling
Select some dried fruit as a sample according to Iran national standard No. 512.
3- Preparing the sample
Prepare the test sample according to Iran national standard No. 1727.
4- Preparing the crucible
Heat the crucible for at least 1 h in a muffle oven set at 550 °C. After cooling in a desiccator, weigh the crucible in the room temperature with accuracy of 0.5 mg (M1).
How to calculate total ash
Weigh about 2 g of the selected sample into a laboratory crucible with accuracy of 0.0001 g (M2). Place the crucible with the sample on the heater to turn the sample into charcoal, then place it in a muffle oven at 550 °C.
After 2 hours, take the container out of the oven and moisten it with water after getting cooled, heat it on Ben Murray then heat again in the muffle oven at a temperature of 550 °C until it reaches a constant mass.
Weigh the crucible after cooling in the desiccator with accuracy of 0.0001g (M3). Repeat the heating, cooling in the desiccator, and weighing operations until the difference between two consecutive weights is higher than 0.0001 g.
Total ash content can be calculated using the following formula:
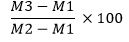
The large number of total ashes indicates the existence of fraud and poor food quality.
How to calculate acid insoluble ash of dried fruit in hydrochloride acid
The laboratorial supplies
- Distilled grade 3 water according to the national Iran national standard No. 1728
- Dilute hydrochloric acid 10 mass %
- Silver nitrate solution (dissolve 10 g of silver nitrate in water and make 100 ml volume)
- Flat-bottom crucible with a capacity of 50 to 100 ml made by platinum, quartz, porcelain, or other immutable materials based on test conditions.
- Filter paper with medium filtering speed
- Electric muffle furnace with a temperature capability of 550±25 °C
- Desiccator
- Scales with an accuracy of 0.0001 grams
- Ben Murray
Acid insoluble ash in hydrochloride acid
The acid-insoluble ash calculation method is used to detect the additives to the main food that are not digestible and are identified as food fraud. This test is important as a quality detection factor.
- Add 15-25 ml HCl to the crucible containing the total obtained ash.
- Boil the solution gently in Ben Murray for about 10 min, then cool it and filter it with a paper filter.
- Wash the crucible and paper filter with hot water until the wash water gets free of hydrochloric acid (about 6 to 8 times).
- Test the presence of HCl with AgNO3.
- If no turbidity was observed in the obtained water from washing by AgNO3, the washing process is complete and stop it.
- Return the remained mass in the paper filter to the crucible and put in oven set at 550 °C.
- Cool the crucible and weigh with accuracy of 0.0001 g.
- Repeat the heating, cooling in the desiccator, and weighing operations until the difference between two consecutive weights is higher than 0.0005 g (M3).
- Acid insoluble ash is calculated by the following equation:
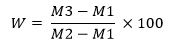
In which,
M1 is the empty crucible weight (g)
M2 is the weight of crucible with sample
M3 is the weight of crucible with sample after heating
Calculate the average of the two measurements and state with accuracy of 0.01.
In addition, acid insoluble ash based on dry mass (W2) is calculated by the following equation:
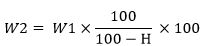
In which,
H is the amount of moisture in the sample based on the mass percentage and W1 is the acid insoluble ash based on mass percentage.
Ref: Identifying food fraud, food quality, and ash of dried fruits







